Amniotic Membrane Market Size and Share Analysis - Growth Trends and Forecast Report 2025-2033
Buy NowAmniotic Membrane Market Trends & Summary
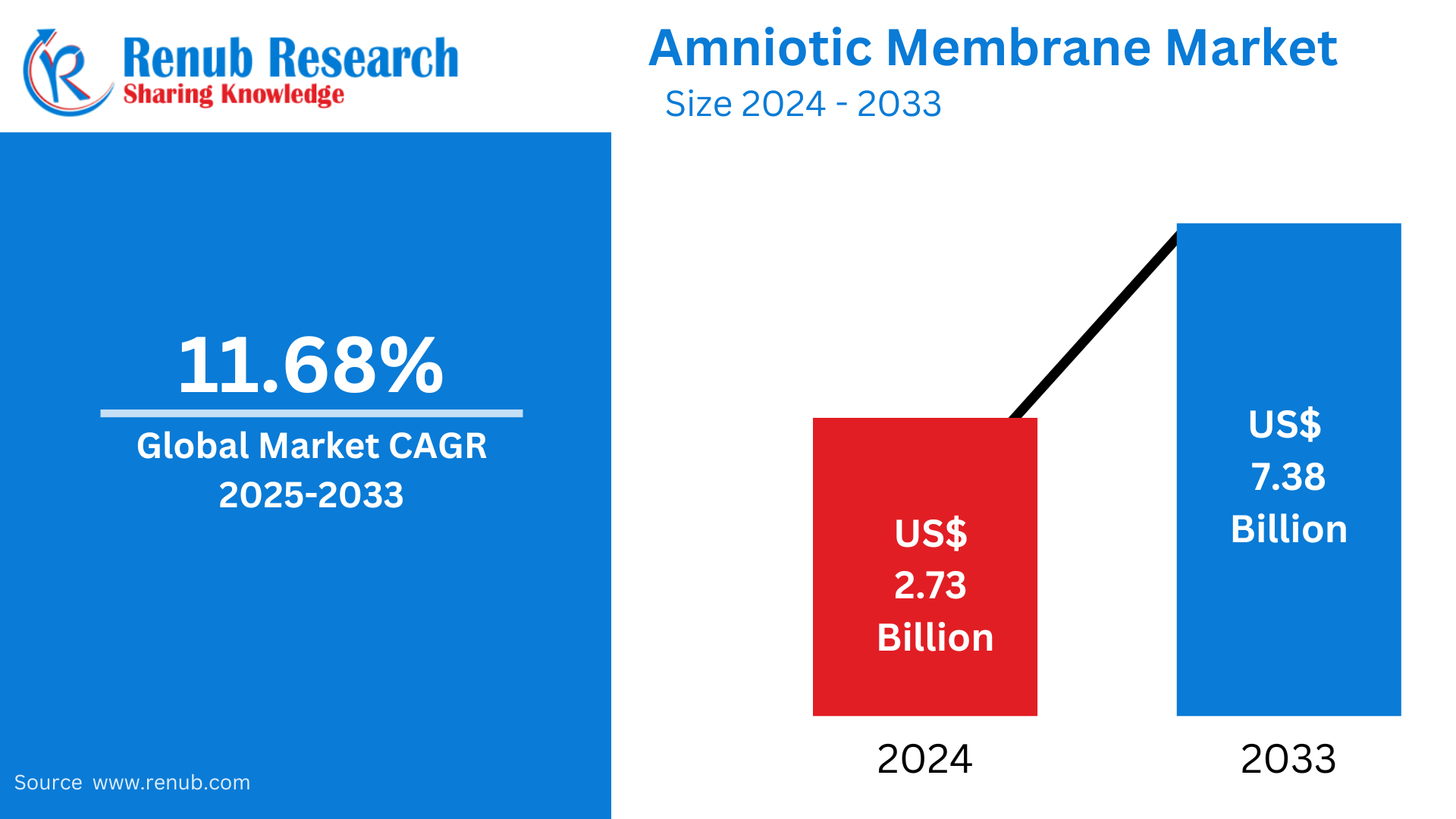
Amniotic Membrane Market is expected to reach US$ 7.38 billion by 2033 from US$ 2.73 billion in 2024, with a CAGR of 11.68% from 2025 to 2033. The market is being driven by a number of significant factors, including the growing popularity of minimally invasive (MI) therapies, the development of various chronic illnesses such as diabetes and rheumatoid arthritis, and the growth of specialist clinics and hospitals.
Amniotic Membrane Global Market Report by Product (Cryopreserved amniotic membranes, Lyophilization Amniotic Membrane), Application (Surgical Wounds, Ophthalmology, Others), End User (Hospitals, Ambulatory Surgical Centers, Specialty Clinics, Research & Academic Institutes), Countries and Company Analysis, 2025-2033
Global Amniotic Membrane Industry Overview
The thick foundation membrane and avascular stromal matrix make up the amniotic membrane, the placenta's deepest layer. Because of its special qualities, this biological tissue—which comes from the placenta—is used in medicine. It lessens tissue fibrosis and scarring and lowers inflammation by regulating inflammatory responses. It speeds up the healing process and has a calming effect on burns and severe wounds. It is frequently utilized in procedures for conditions of the ocular surface, including corneal ulcers, and is well-tolerated and safe with low rejection rates. In order to improve healing and lessen adhesions, it is also used as a biological dressing.
Amniotic membranes are commonly employed in two ways to heal and manage wounds: as a biological bandage or as a surgical graft. When utilized as a graft, these membranes operate as a scaffold to promote re-epithelization. Its anti-inflammatory and anti-scarring qualities are particularly important when burn injuries occur since they help patients feel less uncomfortable and less inflamed. Therefore, it is expected that the growing advantages of amniotic membranes will drive market expansion. Amniotic membrane therapy is frequently used to address conditions affecting the surface of the eye. treatment for a number of conditions, including ocular dystrophy, cataracts, glaucoma, bullous keratopathy, corneal degeneration, bacterial keratitis, corneal ulcers, eyelid reconstruction, and more. There is an enormous demand for these tissue-based products because to the increase in ophthalmology surgery brought on by the global aging population.
Verséa unveiled a point-of-care platform for amniotic membrane transplantation in February 2024. By offering instant diagnostic results and treatment alternatives, this innovation improves patient care in ophthalmology by facilitating quick and efficient testing.
Woo University and NovaBay Pharmaceuticals collaborated in December 2023 to train eyecare providers on the application of amniotic membranes. The collaboration includes a free webinar that offers continuing education credits and covers clinical uses, patient selection, and billing for amniotic membranes, specifically Avenova Allograft from NovaBay, which helps restore the ocular surface.
Growth Drivers for the Amniotic Membrane Market
Technological developments in healthcare
Amniotic membranes are becoming more effective and efficient as a result of the incorporation of cutting-edge technologies. Their application in a variety of medical sectors is increased by modern approaches that guarantee the preservation of vital growth factors and therapeutic capabilities. A promising commercial outlook is being provided by the amniotic membrane's compatibility with innovative therapies like stem cell therapies and three-dimensional (3D) bioprinting. These linkages improve the standard of patient care by enabling more individualized and efficient treatments. In addition, better preservation techniques provide a longer shelf life and more dissemination, which is driving up its uptake in different geographical areas and healthcare systems.
Favorable regulatory framework
Many nations' regulatory agencies are establishing precise and well-defined protocols for the extraction, processing, preservation, and use of amniotic membranes as a result of their recognition of the membrane's medicinal usefulness. These rules maintain product quality and guarantee consistency in procedures. In addition, regulatory bodies keep a close eye on the market to make sure that all applicable laws and guidelines are being followed. The industry's integrity and confidence are preserved by routine audits, inspections, and reporting obligations. Additionally, they are providing supportive policies that promote research and development in the field, such as grants, incentives, and public-private partnerships. Working together with research organizations can spur innovation and introduce cutting-edge goods and treatments to the market.
Growing interest in regenerative medicine
The demand for efficient treatment strategies is growing as a result of the increased incidence of many chronic diseases, such as diabetes and rheumatoid arthritis, which can cause ulcers and chronic wounds. Amniotic membranes promote the healing process and provide a novel solution. In addition, the need for therapies that encourage quicker healing and tissue regeneration is being driven by the world's aging population, which is becoming more and more vulnerable to a number of illnesses. Additionally, the market is being positively impacted by the growing use of amniotic membranes in a variety of medical disciplines, such as dentistry and orthopedics. Furthermore, the amniotic membrane's capacity to promote cellular proliferation and reduce inflammation is propelling its use in other regenerative therapies.
Challenges in the Amniotic Membrane Market
Regulatory Hurdles
Amniotic membrane products are difficult to regulate because of the intricate rules established by organizations such as the FDA. The approval and marketing of amniotic membranes are complicated by the fact that they are subject to both medical device regulations and human tissue laws. Strict regulations for donor screening, tissue processing, labeling, and tracking must be followed by businesses; these regulations can differ depending on the location. These regulatory variations may raise operating expenses and postpone market entry. The strain is further increased by the requirement for strict documentation and quality control. Because of this, companies have a difficult time maintaining compliance while attempting to satisfy consumer needs. Companies hoping to commercially market amniotic membrane-based products worldwide must overcome these regulatory obstacles.
Storage and Shelf-Life Concerns
Due to their limited shelf life as biological materials, amniotic membranes provide storage and transportation issues. For these products to remain viable and functional, certain conditions like freezing or cryopreservation are frequently needed. The supply chain becomes much more expensive and logistically difficult as a result. Furthermore, the broad availability of amniotic membrane products is restricted by the requirement for specialized storage infrastructure, such as temperature-controlled facilities, especially in areas with low resources. These elements raise operating expenses and can make it challenging to continuously supply demand, particularly in rural or developing regions. Improving access and reaching a wider audience need addressing these shelf-life and storage issues.
United States Amniotic Membrane Market
Because of its uses in tissue regeneration, ophthalmology, and wound healing, the amniotic membrane market in the US has grown significantly. The market gains from the rising demand for innovative therapies and regenerative medicine. The increasing frequency of chronic diseases including diabetes and eye disorders, as well as the growing need for minimally invasive therapies, are major factors propelling this expansion. Obstacles include high production costs, regulatory constraints, and moral dilemmas around the use of tissues originating from humans. Notwithstanding these obstacles, the industry is still growing as a result of new technology and rising provider awareness. To get beyond these obstacles and take advantage of the prospects in regenerative medicine, American businesses are spending money on research, product development, and strategic alliances.
In March 2023, Amaro Law Firm released research stating that 7.3 million motor vehicle accidents occur in the United States each year. Therefore, it is projected that over the forecast period, the rising incidence of accidents will increase demand for amniotic membrane.
United Kingdom Amniotic Membrane Market
The market for amniotic membranes in the UK is expanding due to developments in regenerative medicine, namely in the areas of tissue repair, ophthalmology, and wound healing. Demand is rising as a result of a preference for biologic treatments and an increase in the prevalence of chronic disorders including diabetic ulcers and eye problems. Regulatory complexity, high production costs, and restricted donor tissue availability are some of the market's obstacles, though. Market expansion is also impacted by ethical difficulties around the use of tissues originating from humans. Notwithstanding these challenges, the UK market is bolstered by a robust healthcare system, growing knowledge of the uses of amniotic membranes, and continuous studies into their effectiveness. With an emphasis on increasing product accessibility and reducing prices for wider clinical use, there are prospects for additional growth as the use of regenerative therapies increases.
More than 500,000 people in the UK already have venous ulcers of some kind, a number that has doubled over the last ten years, according to data released by The Primary Care Dermatology Society in October 2023. The need for sophisticated wound care treatments, such as amniotic membrane products, has grown dramatically as a result of the increase in instances. These membranes' ability to accelerate healing and lower infection rates makes them an essential part of treating venous leg ulcers, which propels market expansion and provider adoption.
India Amniotic Membrane Market
The growing need for regenerative treatments in ophthalmology, wound healing, and tissue regeneration is driving the amniotic membrane market's expansion in India. This need is being driven by rising rates of chronic diseases like diabetes, diabetic ulcers, and eye ailments. Regulatory complexity, high production costs, and restricted donor tissue availability are some of the market's obstacles, though. Furthermore, acceptance in some areas may be impacted by ethical issues around the usage of items originating from humans. Notwithstanding these obstacles, India's developing healthcare system and rising awareness of cutting-edge treatments are driving the industry. Significant prospects are presented by the growing use of regenerative medicine, and future market growth is anticipated to be driven by continued research into more affordable treatments and easier access to products.
According to Hindustan Times in August 2023, the market for amniotic membranes in India is expanding significantly as a result of a 70% increase in eye infections. Viral and bacterial illnesses have increased as a result of poor sanitary conditions brought on by recent rains and floods. Experts in medicine point to the growing need for amniotic membranes, which have anti-inflammatory and regenerative qualities, in the treatment of conditions affecting the ocular surface.
Saudi Arabia Amniotic Membrane Market
The rising need for cutting-edge regenerative therapies in tissue regeneration, ophthalmology, and wound healing is propelling the amniotic membrane market in Saudi Arabia. The market is expanding due to factors like the increased incidence of chronic illnesses like diabetes and eye disorders. But there are still obstacles like complicated regulations, expensive production, and a shortage of donor tissues. Acceptance may also be influenced by ethical considerations related to the usage of items derived from humans. Notwithstanding these obstacles, the market is expected to develop due to Saudi Arabia's established healthcare system, rising healthcare spending, and rising awareness of regenerative medicine. Further investigation and cooperation with global partners may aid in removing obstacles, promoting growth and opening up amniotic membrane products to a wider range of patients.
This growth is anticipated to be driven by the increase in healthcare spending, which is predicted to reach USD 50.4 billion in 2023, according to a report by the U.S. Department of Commerce and the International Trade Administration. Another important element driving market expansion is the growing number of hospitals and medical professionals.
Amniotic Membrane Market Segments
Product–Market breakup in 2 viewpoints:
- Cryopreserved amniotic membranes
- Lyophilization Amniotic Membrane
Application- Market breakup in 3 viewpoints:
- Surgical Wounds
- Ophthalmology
- Others
End User - Market breakup in 4 viewpoints:
- Hospitals
- Ambulatory Surgical Centers
- Specialty Clinics
- Research & Academic Institutes
Country
- United States
- Canada
- Germany
- United Kingdom
- France
- Italy
- Netherlands
- Spain
- China
- South Korea
- Japan
- India
- Indonesia
- Malaysia
- Argentina
- Brazil
- Mexico
- Colombia
- Saudi Arabia
- South Africa
- Israel
- Australia
- UAE
- ROW
All the Key players have been covered from 4 Viewpoints:
- Overview
- Key Persons
- Recent Developments
- Financial Insights
Company Analysis:
- Stryker Corporation
- Smith & Nephew plc
- Integra LifeSciences Holdings
- MiMedx
- Organogenesis Net
- Wright Medical Group Inc
- Applied Biologics LLC
- FzioMed Inc
- Katena, Inc.
- Tissue-Tech Inc
Report Details:
| Report Features | Details |
| Base Year |
2024 |
| Historical Period |
2020 - 2024 |
| Forecast Period |
2025 - 2033 |
| Market |
US$ Billion |
| Segment Covered |
By Product, By Application, By End User and By Country |
| Countries Covered |
|
| Companies Covered |
|
| Customization Scope |
20% Free Customization |
| Post-Sale Analyst Support |
1 Year (52 Weeks) |
| Delivery Format |
PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on request) |
Key Questions Answered in This Report:
-
What is the projected size of the global amniotic membrane market by 2033?
-
What are the key factors driving the growth of the amniotic membrane market?
-
How do cryopreserved and lyophilized amniotic membranes differ in market usage?
-
Which applications are contributing the most to the amniotic membrane market growth?
-
What are the major challenges faced by the amniotic membrane market?
-
How does the regulatory environment impact the development and commercialization of amniotic membrane products?
-
What role does regenerative medicine play in increasing the adoption of amniotic membranes?
-
How is technological advancement enhancing the effectiveness of amniotic membranes?
-
Which end users are the major consumers of amniotic membrane products?
-
How is the amniotic membrane market performing in key countries like the United States, United Kingdom, India, and Saudi Arabia?
Customization Services available
- Analysis of Market Size and Its Segments
- More Company Profiles (Upto 10 without any additional cost):
- Additional Countries (Other than mentioned Countries):
- Region/Country Specific Reports:
- Market Entry Strategy:
- Region-Specific Market Dynamics:
- Regional Market Share Analysis:
- Trade Analysis:
- Production Insights:
- Others Customized Requests:
For more information contact our analysts.
Need More Assistance?
- Talk to our analysts to get more precious information on the current market trends.
- Include more countries and segments and customize the report based on the final requirement.
- Get a competitive advantage in your industry by knowing the report findings and making a positive impact on your revenues and operations.
- Our analysts are always ready to provide more help and pertinent information if you need any additional assistance.
1. Introduction
2. Research & Methodology
3. Executive Summary
4. Market Dynamics
4.1 Growth Drivers
4.2 Challenges
5. SWOT Analysis
6. Amniotic Membrane Market
7. Market Share Analysis
7.1 By Product
7.2 By Application
7.3 By End User
7.4 By Country
8. Product
8.1 Cryopreserved amniotic membranes
8.2 Lyophilization Amniotic Membrane
9. Application
9.1 Surgical Wounds
9.2 Ophthalmology
9.3 Others
10. End User
10.1 Hospitals
10.2 Ambulatory Surgical Centers
10.3 Specialty Clinics
10.4 Research & Academic Institutes
11. Country
11.1 United States
11.2 Canada
11.3 Germany
11.4 United Kingdom
11.5 France
11.6 Italy
11.7 Netherlands
11.8 Spain
11.9 China
11.10 South Korea
11.11 Japan
11.12 India
11.13 Indonesia
11.14 Malaysia
11.15 Argentina
11.16 Brazil
11.17 Mexico
11.18 Colombia
11.19 Saudi Arabia
11.20 South Africa
11.21 Israel
11.22 Australia
11.23 UAE
11.24 ROW
12. Key Players
12.1 Stryker Corporation
12.1.1 Overview
12.1.2 Key Persons
12.1.3 Recent Development
12.1.4 Revenue
12.2 Smith & Nephew plc
12.2.1 Overview
12.2.2 Key Persons
12.2.3 Recent Development
12.2.4 Revenue
12.3 Integra LifeSciences Holdings
12.3.1 Overview
12.3.2 Key Persons
12.3.3 Recent Development
12.3.4 Revenue
12.4 MiMedx
12.4.1 Overview
12.4.2 Key Persons
12.4.3 Recent Development
12.4.4 Revenue
12.5 Organogenesis Net
12.5.1 Overview
12.5.2 Key Persons
12.5.3 Recent Development
12.5.4 Revenue
12.6 Wright Medical Group Inc
12.6.1 Overview
12.6.2 Key Persons
12.6.3 Recent Development
12.6.4 Revenue
12.7 Applied Biologics LLC
12.7.1 Overview
12.7.2 Key Persons
12.7.3 Recent Development
12.8 FzioMed Inc
12.8.1 Overview
12.8.2 Key Persons
12.8.3 Recent Development
12.9 Katena, Inc.
12.9.1 Overview
12.9.2 Key Persons
12.9.3 Recent Development
12.10 Tissue-Tech Inc
12.10.1 Overview
12.10.2 Key Persons
12.10.3 Recent Development
Reach out to us
Call us on
USA: +1-478-202-3244
INDIA: +91-120-421-9822
Drop us an email at
info@renub.com