Balloon Catheter Market Global Report by Product Type (Normal, Drug Eluting, Cutting, Scoring, Stent Graft, and Others), Indication (Coronary Artery Disease and Peripheral Vascular Disease), Raw Materials (Polyurethane, Nylon, and Others), End-User (Hospitals, Clinics, Ambulatory Surgical Centers, and Diagnostic Centers), Countries and Company Analysis 2025-2033
Buy NowGlobal Balloon Catheter Market Size
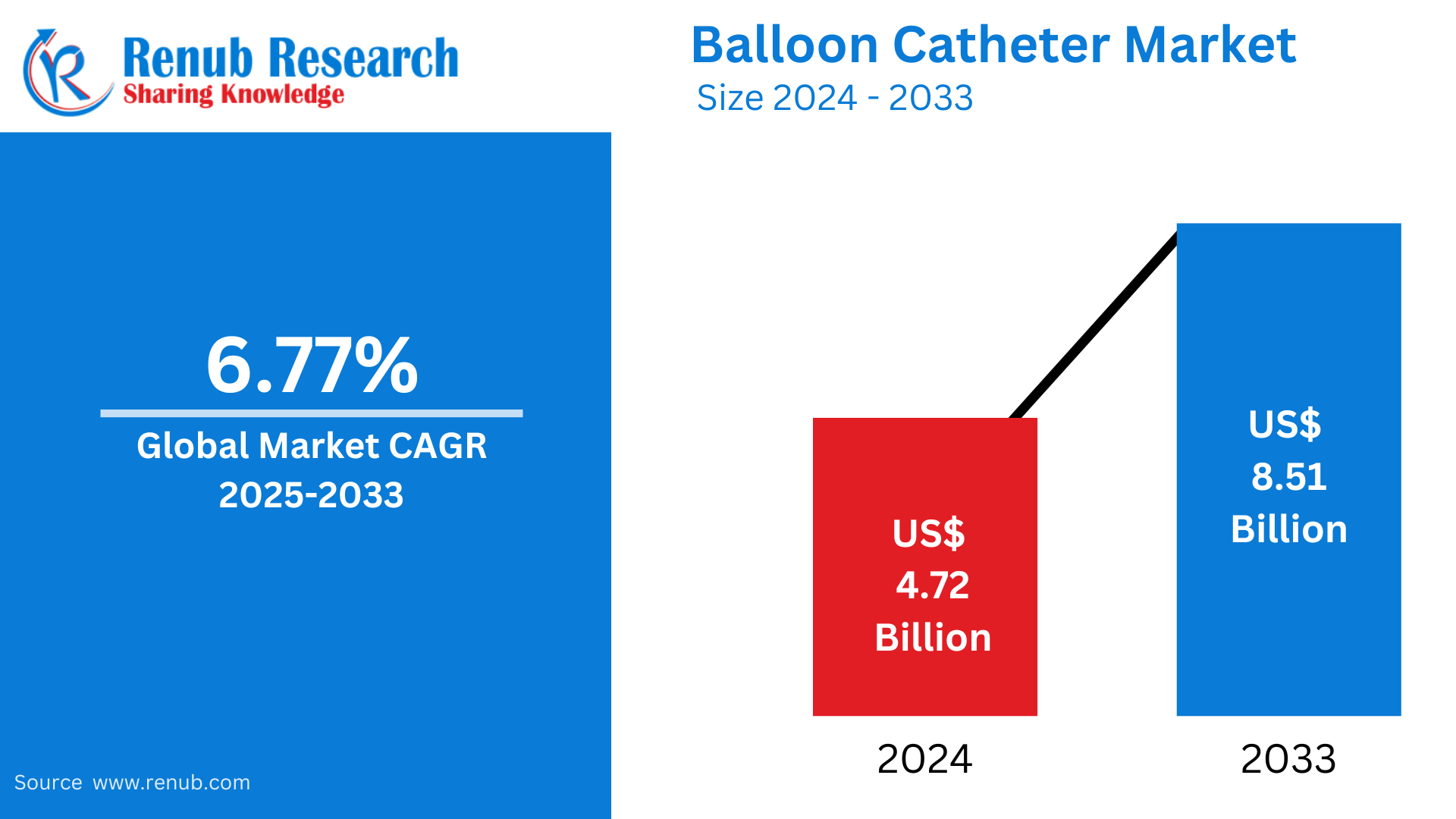
The global balloon catheter market is expected to reach US$8.51 billion in 2033, which is growing from US$4.72 billion in 2024 at a CAGR of 6.77% from 2025 to 2033. This growth will be driven by the rise in incidences of cardiovascular and peripheral vascular diseases, increasing adoption of minimally invasive procedures, and advancements in catheter technology. Also, the growing aging population along with the demand for efficient diagnostic and therapeutic tools increase the market.
Global Balloon Catheter Market Outlooks
A balloon catheter is a medical device that specifically is used in minimally invasive procedures to treat blockages or narrowings in blood vessels and other body pathways. It's a thin, flexible tube, with an inflatable balloon located at the tip, which gets inserted into the affected area then expanded to restore proper flow or create space. This device is a cornerstone in treating cardiovascular diseases, especially in procedures such as angioplasty, which helps widen narrowed or obstructed arteries, improving blood flow to the heart and reducing risks associated with coronary artery disease.
Balloon catheters are used for a variety of applications other than cardiology. They remove limb artery blockages in peripheral vascular procedures, thus ensuring adequate blood circulation. They also help in non-vascular applications like the dilation of urinary tract or esophagus strictures. Some balloon catheters are coated with therapeutic drugs that allow for the direct application of drugs to the affected region, thus improving treatment outcomes. The versatility, precision, and minimally invasive nature of balloon catheters make them indispensable in the practice of modern medicine.
Growth Driver in the Balloon Catheter Market
Increasing Prevalence of Cardiovascular Diseases
There is a growing prevalence of cardiovascular diseases (CVDs) worldwide. Most cardiovascular diseases like coronary artery disease and peripheral artery disease require minimally invasive treatments like angioplasty, where balloon catheters are critical. Increased levels of sedentary lifestyle, unhealthy diet, and growing population all have led to increased incidences of CVD, ensuring a constant demand for the related devices. Early diagnosis and treatment of CVD with evolving healthcare systems have expanded advanced balloon catheter technologies adoption worldwide and further spurred the market and improved patient outcomes. The 2022 National Health Survey estimated that around 1.3 million Australians aged 18 and above (6.7% of adults) reported one or more conditions related to heart, stroke, and vascular disease. This included 600,000 adults with coronary heart disease at 3.0%. Cardiovascular disease was more common in men (7.6%) than women (5.8%) and affected about 28% of adults aged 75 and over.
Advancements in Catheter Technology
Technological advancements in balloon catheter design and materials improve efficiency, safety, and range of applications. Innovations such as drug-coated balloon catheters, which deposit medication directly at the treatment site, have increased their ability to prevent restenosis. Improved flexibility, smaller diameters, and better imaging compatibility have also broadened their application in complex procedures. These advancements reduce complications, increase procedural success rates, and make balloon catheters more attractive to practitioners, which in turn spur market growth. In September 2024, Biotronik, Switzerland, rolled out the FlowGuide and Guidion Short guide extension catheters in selected countries that accept the CE Mark.
Increasing adoption of minimally invasive surgery techniques is the most relevant growth driver for the balloon catheter market. Increasing numbers of patients and healthcare providers choose such procedures due to short periods for recovery, low risk for complications, and shorter stays at hospitals. In such procedures as balloon angioplasty and other endovascular interventions, the balloon catheter is most relevant. Advances in imaging techniques as well as in the catheters' design improve the outcome and accessibility of the less invasive procedures, hence fostering the demand for balloon catheters across a broader array of medical conditions and wider geographic markets.
Challenges in the Balloon Catheter Market
High Costs and Limited Accessibility
The high cost of balloon catheters, especially advanced types such as drug-coated or cutting balloon catheters, is a significant challenge in the global market. These costs can limit accessibility, especially in low- and middle-income countries with constrained healthcare budgets. The expenses of training healthcare professionals and acquiring advanced imaging systems required for procedures further complicate adoption. The patients in the underserved areas may not have access to these devices, and hence, they cannot be treated on a large scale. Developing cost-effective alternatives and improving the healthcare infrastructure is important to overcome this barrier and ensure equitable access to these life-saving technologies.
Stringent Regulatory Approvals
The regulatory approval process for balloon catheters is long and complex because of the stringent testing required to ensure safety and efficacy. Manufacturers must comply with diverse regulatory standards across regions, which can delay product launches and increase development costs. Frequent updates to regulatory frameworks, such as changes in EU MDR guidelines, add additional compliance challenges. These hurdles can discourage smaller companies from entering the market and slow innovation. Addressing these challenges requires streamlined regulatory processes and enhanced collaboration between manufacturers and regulatory bodies to facilitate faster yet safer device approvals.
United States Balloon Catheter Market
United States balloon catheter market in North America is dominated by the United States, primarily due to high prevalence of cardiovascular diseases, better healthcare infrastructure, and increased usage of minimally invasive procedures. The presence of leading companies in medical devices and investments in R&D also drives the market. Rising awareness about heart health and access to advanced technologies make the U.S. a key market for innovations like drug-coated balloon catheters. However, high healthcare costs and regulatory challenges can impact market expansion. The ongoing focus on improving patient outcomes and expanding healthcare access ensures sustained U.S. balloon catheter market growth.
Germany Balloon Catheter Market
Germany leads the balloon catheter market in Europe, owing to its robust healthcare system and intense focus on medical innovation. The country’s aging population and increasing cardiovascular and peripheral vascular disease cases drive the demand for balloon catheters. Germany’s emphasis on adopting cutting-edge technologies, including drug-eluting catheters, has bolstered the market. Additionally, favorable reimbursement policies and collaboration between healthcare providers and manufacturers support growth. However, stringent EU MDR regulations are a challenge. But still, Germany is a central location for advanced medical devices; thus, it will maintain its dominance in the European balloon catheter market. Oct 2022 – Biosense Webster, a Johnson & Johnson MedTech company, introduced HELIOSTAR™ Balloon Ablation Catheter in Europe. It is the first catheter designed for catheter-based cardiac electrophysiological mapping and cardiac ablation when used with a compatible multi-channel RF generator.
China Balloon Catheter Market
The Asia-Pacific market for balloon catheters, especially China, is expected to see rapid growth in demand because of cardiovascular diseases from the current lifestyle and expanded healthcare availability. Government initiatives to enhance medical infrastructure, and promote domestic productions of the medical devices, are assisting in propelling the market towards growth. Local companies continue to make investments in cost-efficient balloon catheters to bridge the supply gap for the country's massive population. However, there exist challenges such as significant disparities between urban and rural healthcare facilities. With present reforms and technological advancements underway, China is expected to emerge as a major stakeholder in the global market for balloon catheters. In March 2024, TekniPlex Healthcare will present its new catheter design and manufacturing capabilities in China.
Brazil Balloon Catheter Market
Brazil is the balloon catheter market leader in Latin America. An increasing burden of cardiovascular diseases and growing awareness about minimally invasive procedures are driving the market. Public and private sector investments in healthcare infrastructure have improved access to advanced medical technologies. Angioplasty and related treatments have expanded the use of balloon catheters in Brazil. Economic instability and uneven healthcare distribution, however, remain challenges. Despite these obstacles, government efforts to enhance healthcare coverage and rising medical tourism are expected to fuel Brazil's balloon catheter market growth. For the first time, September 2023, Endovastec™'s Reewarm™ PTX Drug-Coated Balloon (DCB) Catheter was successfully implanted in Brazil and marks its growing presence in international markets.
Saudi Arabia Balloon Catheter Market
Balloon catheters in the Middle East and Africa find a promising emerging market in Saudi Arabia. Governmental efforts to enhance healthcare services through Vision 2030 form the backbone of the Saudi market. The increasing prevalence of cardiovascular diseases and obesity-related conditions boosts demand for minimally invasive treatments like angioplasty. Investments in advanced medical infrastructure and the growing adoption of innovative medical devices, including drug-coated balloon catheters, drive market expansion. However, reliance on imports and the high cost of advanced devices pose challenges. The healthcare reforms in continuous progress and partnerships with world-class manufacturers will further increase availability and lower the cost of balloon catheters in Saudi Arabia. March 2021, MicroPort Medical (Group) Co. Ltd., Shanghai recently got the approval for four products from the Saudi Food and Drug Authority- Firehawk Rapamycin Target Eluting Coronary Stent System, Firehawk Liberty Rapamycin Target Eluting Coronary Stent System, Firefighter PTCA Balloon Catheter, and Firefighter NC PTCA Balloon Catheter.
Product Type – Market breakup from 6 viewpoints:
1. Normal
2. Drug Eluting
3. Cutting
4. Scoring
5. Stent Graft
6. Others
Indication – Market breakup from 2 viewpoints:
1. Coronary Artery Disease
2. Peripheral Vascular Disease
Raw Materials – Market breakup from 3 viewpoints:
1. Polyurethane
2. Nylon
3. Others
End-User – Market breakup from 4 viewpoints:
1. Hospitals
2. Clinics
3. Ambulatory Surgical Centers
4. Diagnostic Centers
Country – Market breakup from 26 Country Global Balloon Catheter Industry viewpoints:
1. North America
1.1 United States
1.2 Canada
2. Europe
2.1 France
2.2 Germany
2.3 Italy
2.4 Spain
2.5 United Kingdom
2.6 Belgium
2.7 Netherland
2.8 Turkey
3. Asia Pacific
3.1 China
3.2 Japan
3.3 India
3.4 South Korea
3.5 Thailand
3.6 Malaysia
3.7 Indonesia
3.8 Australia
3.9 New Zealand
4. Latin America
4.1 Brazil
4.2 Mexico
4.3 Argentina
5. Middle East & Africa
5.1 Saudi Arabia
5.2 UAE
5.3 South Africa
6. Rest of the World
All companies have been covered from 3 viewpoints:
• Overview
• Recent Developments
• Revenue
Company Analysis:
1. Abbott Laboratories
2. Becton Dickinson and Company
3. Cardinal Health
4. Teleflex Incorporated
5. Medtronic Plc.
6. Johnson and Johnson
7. Edwards Lifesciences Corporation
8. Stryker Corporation
9. Smith & Nephew
Report Details:
| Report Features | Details |
| Base Year |
2024 |
| Historical Period |
2020 - 2024 |
| Forecast Period |
2025 - 2033 |
| Market |
US$ Billion |
| Segment Covered |
Product Type, Lead Type, End User and Countries |
| Countries Covered |
|
| Companies Covered |
|
| Customization Scope |
20% Free Customization |
| Post-Sale Analyst Support |
1 Year (52 Weeks) |
| Delivery Format |
PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on request) |
Key Questions Answered in This Report:
-
What is the projected market size of the global balloon catheter market by 2033?
-
What are the major drivers propelling the growth of the balloon catheter market?
-
How is the increasing prevalence of cardiovascular diseases impacting market demand?
-
What role do technological advancements play in balloon catheter development?
-
What are the major challenges hindering the global balloon catheter market?
-
How does the regulatory environment affect balloon catheter market expansion?
-
Which product types dominate the global balloon catheter market?
-
Which raw materials are most commonly used in manufacturing balloon catheters?
-
What is the market share of different end-user segments like hospitals, clinics, and ambulatory centers?
-
Which countries are leading the balloon catheter market across different regions?
-
How is the adoption of minimally invasive procedures influencing balloon catheter usage?
-
What recent product launches or innovations have shaped the market landscape?
-
What is the future outlook for balloon catheters in non-vascular applications?
-
How are emerging markets like China, Brazil, and Saudi Arabia contributing to global market growth?
-
What competitive strategies are adopted by key players in the balloon catheter industry?
Customization Services available
- Analysis of Market Size and Its Segments
- More Company Profiles (Upto 10 without any additional cost):
- Additional Countries (Other than mentioned Countries):
- Region/Country Specific Reports:
- Market Entry Strategy:
- Region-Specific Market Dynamics:
- Regional Market Share Analysis:
- Trade Analysis:
- Production Insights:
- Others Customized Requests:
For more information contact our analysts.
Need More Assistance?
- Talk to our analysts to get more precious information on the current market trends.
- Include more countries and segments and customize the report based on the final requirement.
- Get a competitive advantage in your industry by knowing the report findings and making a positive impact on your revenues and operations.
- Our analysts are always ready to provide more help and pertinent information if you need any additional assistance.
1. Introduction
2. Research Methodology
3. Executive Summary
4. Market Dynamics
4.1 Growth Drivers
4.2 Challenges
5. Global Balloon Catheter Market
6. Market Share
6.1 By Product Type
6.2 By Indication
6.3 By Raw Material
6.4 By End Users
6.5 By Countries
7. Product Type
7.1 Normal Balloon Catheter
7.2 Drug Eluting Balloon Catheter
7.3 Cutting Balloon Catheter
7.4 Scoring Balloon Catheter
7.5 Stent Graft Balloon Catheter
7.6 Others
8. Indication
8.1 Coronary Artery Disease
8.2 Peripheral Vascular Disease
9. Raw Material
9.1 Polyurethane
9.2 Nylon
9.3 Others
10. End Users
10.1 Hospitals
10.2 Clinics
10.3 Ambulatory Surgical Centers
10.4 Diagnostic Centers
11. Countries
11.1 North America
11.1.1 United States
11.1.2 Canada
11.2 Europe
11.2.1 France
11.2.2 Germany
11.2.3 Italy
11.2.4 Spain
11.2.5 United Kingdom
11.2.6 Belgium
11.2.7 Netherland
11.2.8 Turkey
11.3 Asia Pacific
11.3.1 China
11.3.2 Japan
11.3.3 India
11.3.4 South Korea
11.3.5 Thailand
11.3.6 Malaysia
11.3.7 Indonesia
11.3.8 Australia
11.3.9 New Zealand
11.4 Latin America
11.4.1 Brazil
11.4.2 Mexico
11.4.3 Argentina
11.5 Middle East & Africa
11.5.1 Saudi Arabia
11.5.2 UAE
11.5.3 South Africa
11.6 Rest of the World
12. Porter’s Five Forces Analysis
12.1 Bargaining Power of Buyers
12.2 Bargaining Power of Suppliers
12.3 Degree of Rivalry
12.4 Threat of New Entrants
12.5 Threat of Substitutes
13. SWOT Analysis
13.1.1 Strength
13.1.2 Weakness
13.1.3 Opportunity
13.1.4 Threat
14. Key Players Analysis
14.1 Abbott Laboratories
14.1.1 Overview
14.1.2 Recent Development
14.1.3 Revenue
14.2 Becton Dickinson And Company
14.2.1 Overview
14.2.2 Recent Development
14.2.3 Revenue
14.3 Cardinal Health
14.3.1 Overview
14.3.2 Recent Development
14.3.3 Revenue
14.4 Teleflex Incorporated
14.4.1 Overview
14.4.2 Recent Development
14.4.3 Revenue
14.5 Medtronic Plc.
14.5.1 Overview
14.5.2 Recent Development
14.5.3 Revenue
14.6 Johnson & Johnson
14.6.1 Overview
14.6.2 Recent Development
14.6.3 Revenue
14.7 Edwards Lifesciences Corporation
14.7.1 Overview
14.7.2 Recent Development
14.7.3 Revenue
14.8 Stryker Corporation
14.8.1 Overview
14.8.2 Recent Development
14.8.3 Revenue
14.9 Smith & Nephew
14.9.1 Overview
14.9.2 Recent Development
14.9.3 Revenue
Reach out to us
Call us on
USA: +1-478-202-3244
INDIA: +91-120-421-9822
Drop us an email at
info@renub.com