United States Generic Drugs Market to be USD 210 Billion by 2024
19 Jul, 2018
Get Free Customization in This Report
As per Renub Research analysis United States Generic Drugs Market is anticipated to be more than USD 210 Billion by the year 2024. It has approximately 80% share of total dispensing prescriptions. Now most of the physicians and hospitals like prescribe generic drugs as compared to branded drugs.
What is a Generic Drug?
It is a Pharmaceutical drug that has the same chemical composition as patented drug. It has the same Active Pharmaceutical Ingredient (API) as original, but may have different manufacturing process, excipients, color, taste and packaging. It is only allowed when the patent(s) of on original drug is expired.
Request a free sample copy of the report: https://www.renub.com/contactus.php
Generic Drugs Market in United States
In 2007, the Food and Drug Administration (FDA) launched the Generic Initiative for Value and Efficiency (GIVE) to increase the number and a variety of generic products by modernizing and streamlining the approval process for generic drugs in US. For making a generic drug, pharmaceutical companies are required to file an Abbreviated New Drug Application (ANDA) with FDA.
Why Generic Drugs are more affordable than Branded Drugs?
A branded drug has to go through many phases before coming into the market; initial phase is drug discovery, target identification and validation then Primary Screening, Secondary Screening, Lead Optimization, Pre-Clinical Animal Studies and various other tests. As new drug development is a complex process and involves various R & D of chemicals, but clinical trials and regulatory processes are also required to be approved for human use of drugs.
How Drugs Development Process Works?
According to some studies only 5 out of 5000 preclinical drugs are tested on the human for medical use out of those 5 drugs only 1 is approved by FDA. There are various stages involved in drug development.
Drug Discovery and Target Validation: In this stage drug development company chooses a molecule, such as a protein or gene and test it with a drug. After testing multiple drugs only a few are selected for next stage.
Preclinical Testing: It is divided into subcomponents: in vitro and in vivo testing. In vitro testing examines drug molecules interactions in test tubes in the lab setting. In vivo testing involves test is being done on animal models and other living cell cultures. These tests may take several years before going to next stage.
Investigational New Drug Application Filing: At this stage an application is submitted to FDA before beginning human clinical trials. At this point FDA will scrutinize the outcomes of preclinical testing, side effects and other safety measures for an experimental drug. When IND got approved by FDA then it can move onto human trials. After IND approval a patented drugs' 20 year exclusivity period begins.
Client can Purchase this Report in Sections from below link:
Access full Research: https://www.renub.com/united-states-off-label-drugs-market-and-forecast-51-p.php
Phase 1 Clinical Studies: At this stage testing involves a small group of healthy people from a dozen to a few in numbers and safety is taken as a primary concern. Now all the side effect of drug, how a drug is absorbed and elimination from the body is tested.
Phase 2 Clinical Studies: After getting positive outcomes from Phase1, Phase 2 begins in this phase the number of patients grows from a few dozen to 100 or more patients who are suffering from disease. Safety is also a big concern at this point and short term side effects are also monitored closely.
Phase 3 Clinical Studies: At this stage the number of patients for drug testing grow from a few hundred to thousands. It is the longest and costliest of all components of development process.
New Drug Application Filing: At this point application is filed to FDA, with all the research and safety data of initial stages having tens of thousands to 100,000 or more pages. After getting affirmation from FDA, Prescription Drug User Fee Act (PDUFA) date is set 10 months.
PDUFA Date and Decision: FDA will wait for PDUFA date for its decision. FDA has 3 choices: it can approve a drug, it can deny a drug or it can request additional information by sending a Complete Response Later (CRL). If a drug is approved by FDA, now it becomes immediately available for commercial production.
Phase 4 Clinical Studies: After 8 steps an approved drug can used for medical purpose, but it doesn't mean it is not under scrutiny of FDA, FDA can request long-term safety measures, side effect and adverse effect of drug.
Key Topics Covered :
1. Executive Summary
2. United States Pharmaceutical Market
2.1 Branded Pharmaceutical Market
2.2 Generics Pharmaceutical Market
3. United States Generic Drugs Saving
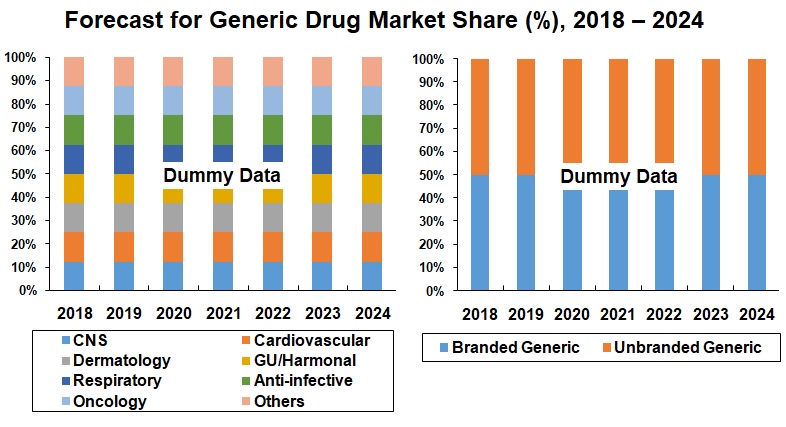
4. Market Share – United States Pharmaceutical
4.1 Branded vs. Generics Drugs
4.2 Branded Generic vs. Unbranded Generic Drug
4.3 Volume Penetration – Generic vs. Branded Drugs
4.4 Therapeutic Application
5. United States Generic Drugs Market (2010 – 2024)
5.1 Branded Generic Drugs Market
5.2 Unbranded Generic Drugs Market
6. Therapeutic Application – United States Generic Drugs Market (2010 – 2024)
6.1 Central Nervous System (CNS)
6.2 Cardiovascular
6.3 Dermatology
6.4 Genitourinary / Hormonal Drugs
6.5 Respiratory
6.6 Anti-infective
6.7 Oncology
6.8 Others
7. Teva Pharmaceutical Industries Ltd - Company Analysis
7.1 Product Launch
7.2 Financial Insight
8. Mylan N.V. - Company Analysis
8.1 Product Launch
8.2 Financial Insight
9. Sandoz Inc. - Company Analysis
9.1 Product Launch
9.2 Financial Insight
10. Endo Pharmaceuticals - Company Analysis
10.1 Product Launch
10.2 Financial Insight
11. Lupin Limited - Company Analysis
11.1 Product Launch
11.2 Financial Insight
12. Dr Reddy's - Company Analysis
12.1 Business Portfolio
12.2 Financial Insight
13. Sun Pharmaceutical Industries Ltd. - Company Analysis
13.1 Product Launch
13.2 Financial Insight
14. Others
14.1 Financial Insight
15. Growth Driver
15.1 Rising Geriatric Population of United States
15.2 Generic Drugs Cost less than Brand Name Drugs
15.3 Rising Incidence of non-communicable Diseases
16. Challenges
16.1 Lack of Regulation & Information
16.2 Long Halt for Expiration of Patent Drug
About Us
Renub Research is a Market Research and Consulting Company. We have more than 10 years of experience especially in international Business-to-Business Researches, Surveys and Consulting. We provide wide range of business research solutions that helps companies in making better business decisions. Our clients rely on our market analysis and data to make informed knowledgeable decisions. Our pertinent analysis helps consultants, bankers and executives to make informed and correct decisions.
Contact Us
Renub Research
Phone: +1-678-302-0700
Email: info@renub.com
Website: https://www.renub.com
Follow us on LinkedIn: https://www.linkedin.com/company/renub-research
Reach out to us
Call us on
USA: +1-678-302-0700
INDIA: +91-120-421-9822
Drop us an email at
info@renub.com